INTERCEPT PLATELETS
Delivers Value
INTERCEPT® Platelets Deliver Value and Operational Efficiencies
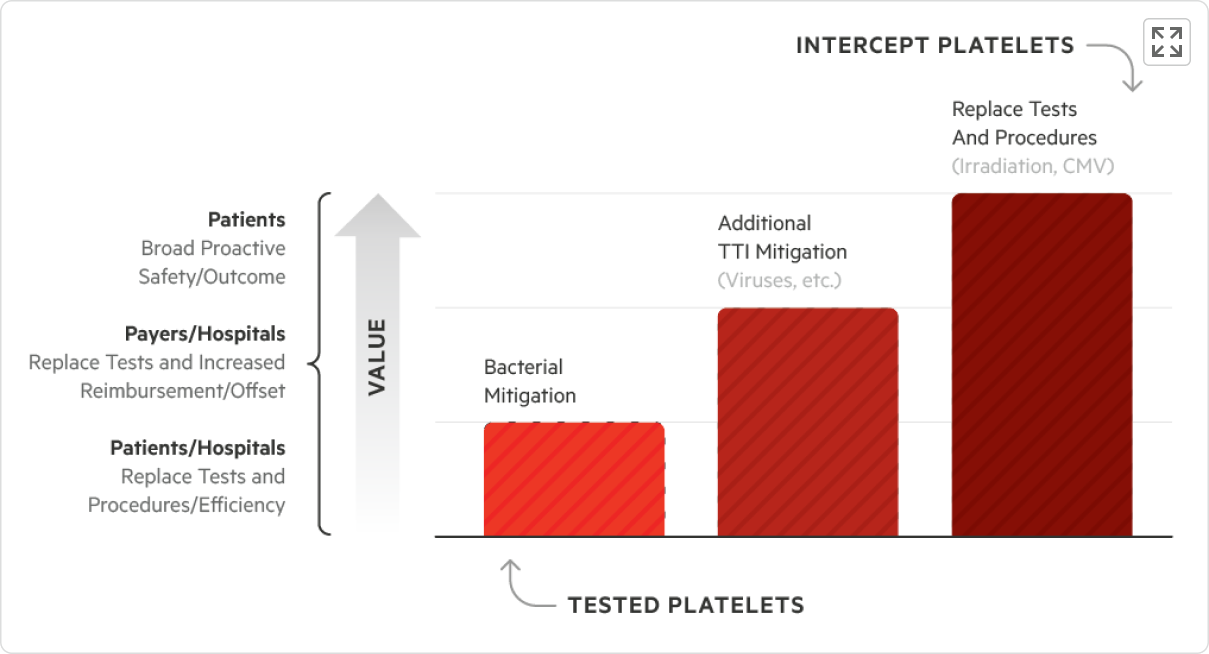
Efficiencies gained and transfusion-transmitted infection (TTI) risk reduction with platelets treated with the INTERCEPT Blood System Pathogen Reduction System (INTERCEPT Platelets) may translate to economic benefits:
- Receipt of transfusion-ready platelets with the ability to use INTERCEPT Platelets in place of certain tests and/or procedures, such as bacterial detection, CMV serology, and irradiation1-5
- Reduced TTI risk* may save costs on associated treatments and investigations
- Avoidance of false positives and associated recalls, may save time, resources, and platelet units
- Minimized platelet contamination risk, which could jeopardize critical procedures such as stem cell transplantations
Also, with PR platelets, the need for irradiation prior to issue is no longer present. This allows us to distribute platelets more quickly to patients in need.
Dr. Shannon Walker, MD
Assistant Professor, Transfusion Medicine & Pediatric Hematology/Oncology
Department of Pathology, Microbiology & Immunology and Pediatrics
Vanderbilt University Medical Center

You may also be interested in
*There is no pathogen reduction process that has been shown to eliminate all pathogens. Certain non-enveloped viruses (e.g., HAV, HEV, B19, and poliovirus) and Bacillus cereus spores have demonstrated resistance to the INTERCEPT process. For a full list of pathogens, see Package Insert.
References:
- Bacterial Risk Control Strategies for Blood Collection Establishments and Transfusion Services to Enhance the Safety and Availability of Platelets for Transfusion: Guidance for Industry. US FDA; December 2020.
- Recommendations for Reducing the Risk of Transfusion-Transmitted Malaria”, FDA Guidance for Industry. December 2022.
- “Recommendations for Reducing the Risk of Transfusion-Transmitted Babesiosis”, FDA Guidance for Industry. May 2019.
- Harm SK, et al. Transfusion. 2018 Apr; 58(4):938-942.
- Ruby KN, et al. Transfusion. 2018 Jul; 58(7):1665-1669.