WHEN MINUTES MATTER
What’s in the Bag?
INTERCEPT® Fibrinogen Complex is an enriched source of key constituents in effective hemostasis for the treatment and control of hemorrhage associated with fibrinogen deficiency.
INTERCEPT® Fibrinogen Complex is a pathogen reduced cryoprecipitated fibrinogen complex derived from the cryoprecipitation* of human plasma.
INTERCEPT Fibrinogen Complex is produced from the INTERCEPT Blood System for Cryoprecipitation to make an enriched source of key constituents in effective hemostasis1-3:
- Fibrinogen
- Factor XIII
- Von Willebrand Factor
Functional levels of these key constituents correlate with the risk of bleeding, morbidity and mortality.4-6
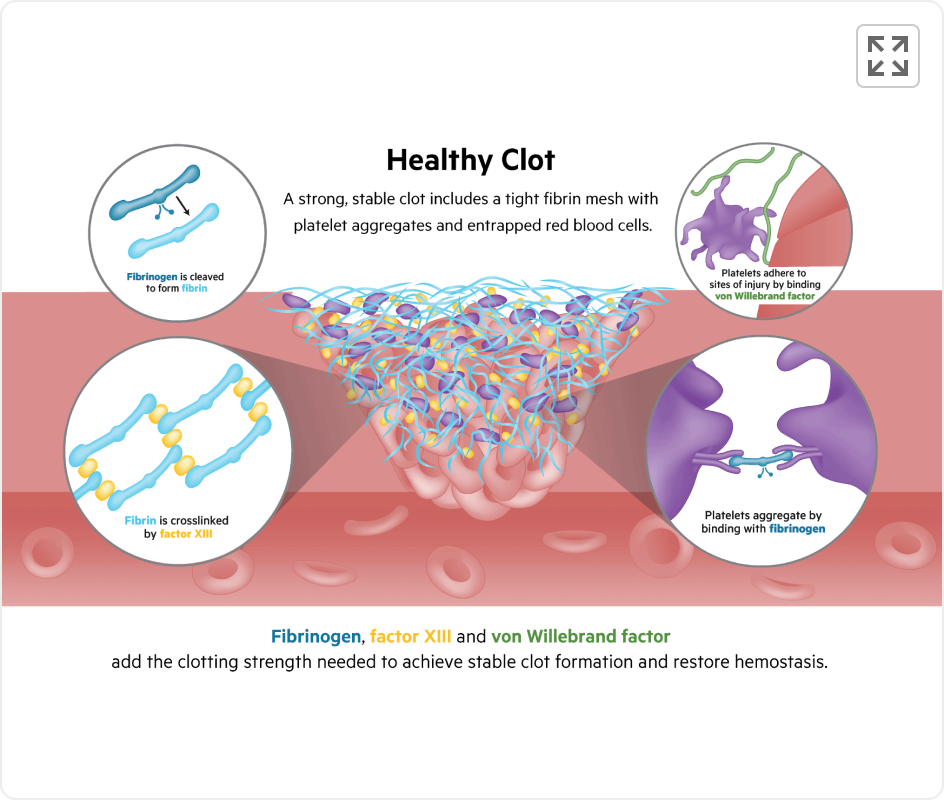
Healthy Clot
Platelets and red blood cells are tightly bound to the vessel wall and each other by fibrinogen, fibrin, factor XIII, and von Willebrand factor, key constituents of hemostasis7
Fibrinogen, Factor XIII and von Willebrand factor add the clotting strength needed to achieve stable clot formation and restore hemostasis.
The INTERCEPT Blood System provides broad spectrum pathogen reduction of the plasma used to derive the INTERCEPT Fibrinogen Complex allowing it to be stored transfusion ready, at room temperature, post-thaw, for up to 5 days.8
INTERCEPT Fibrinogen Complex retains in vitro functional characteristics 5 days post-thaw.8
| FC15 Characteristics | Result at Thaw | Result at 5 days Post-Thaw |
|
Volume |
147 ± 7 [136 – 155] |
Not measured |
|
Fibrinogen content |
1556 ± 248 [1209 – 1913] |
1435 ± 206 [1129 – 1760] |
|
Factor VIII content |
407 ± 78 [333 – 567] |
367 ± 78 [276 – 518] |
|
Factor XIII antigen |
17.2 ± 3.3 [13.6 – 24.3] |
16.0 ± 2.2 [12.4 – 18.8] |
|
vWF ristocetin |
720 ± 210 [423 – 977] |
702 ± 215 [399 – 961] |
| vWF Antigen (IU) |
979 ± 132 [848 – 1269] |
985 ± 147 [869 – 1327] |
Mean ± Standard Deviation (SD) [range], n = 8
#INTERCEPT Fibrinogen Complex sample was derived from 4 whole blood-derived plasma inputs
You may also be interested in
*Cryoprecipitation is the process of using cold temperature to cause soluble factors to fall out of solution, also known as precipitation, allowing for separation and concentration of these factors.
References:
- Levy JH, Welsby I, et al. Fibrinogen as a therapeutic target for bleeding: a review of critical levels and replacement therapy. Transfusion 2014;54(5):1389-1405; quiz 1388.
- Schroeder V, Kohler HP. Factor XIII: Structure and Function. Semin Thromb Hemost 2016;42(4):422-428.
- Peyvandi, F. Diagnosis and management of patients with von Willebrand’s disease in Italy: an Expert Meeting Report. Blood Transfus 2018;16(4):326-328.
- Rourke C, Curry N, Khan S, et al. Fibrinogen levels during trauma hemorrhage, response to replacement therapy, and association with patient outcomes. Journal of thrombosis and haemostasis : JTH 2012;10:1342-51.
- Peyvandi F, Kouides P, Turecek PL, Dow E, Berntorp E. Evolution of replacement therapy for von Willebrand dicsease: From plasma fraction to recombinant von Willebrand factor. Blood reviews 2019.
- von Rappard S, Hinnen C, Lussmann R, Rechsteiner M, Korte W. Factor XIII Deficiency and Thrombocytopenia Are Frequent Modulators of Postoperative Clot Firmness in a Surgical Intensive Care Unit. Transfus Med Hemother 2017;44:85-92.
- Chapin JC, Hajjar KA. Fibrinolysis and the control of blood coagulation. Blood reviews 2015;29:17-24.
- INTERCEPT Blood System for Cryoprecipitation for the manufacturing of Pathogen Reduced Cryoprecipitated Fibrinogen Complex Package Insert.